List of services
Support 24/24
Recents post
Consulting for Kim Tin Medical Supplies Co., Ltd (Kinyee Vietnam) to build and get ISO 13485:2016 certification
Date post: 07-08-2020 | Admin
ISO 13485 on medical devices and related services testing, conformity with standards and regulations, declaration of eligibility to manufacture medical devices, CE, FDA
.jpg)
In the heat of the Covid season and will be the demand after Covid with the process of moving production to Vietnam from China, we are consulting and supporting Kim Tin Medical Supplies Co., Ltd (Kinyee Vietnam) to build and take ISO 13485:2016 certification.
.jpg)
This is a standard that specifies requirements for a quality management system to be applied in facilities that provide medical devices and related services to ensure product availability. Meet customer requirements and legal requirements
Post in the same category
- ( Date post: 15-11-2021 )
- ISO Solutions-ISO certification consulting in the season of Covid-19 ( Date post: 10-06-2021 )
- The importance of ISO Management system in small and medium companies in Vietnam ( Date post: 10-05-2021 )
- ISO Solutions Consulting for ISO 45001 Certification at Minh Tan Environment Company ( Date post: 19-04-2021 )
- Consulting for ISO 9001:2015 certification at Minh Phuong Manufacturing and Trading Co., Ltd ( Date post: 31-03-2021 )






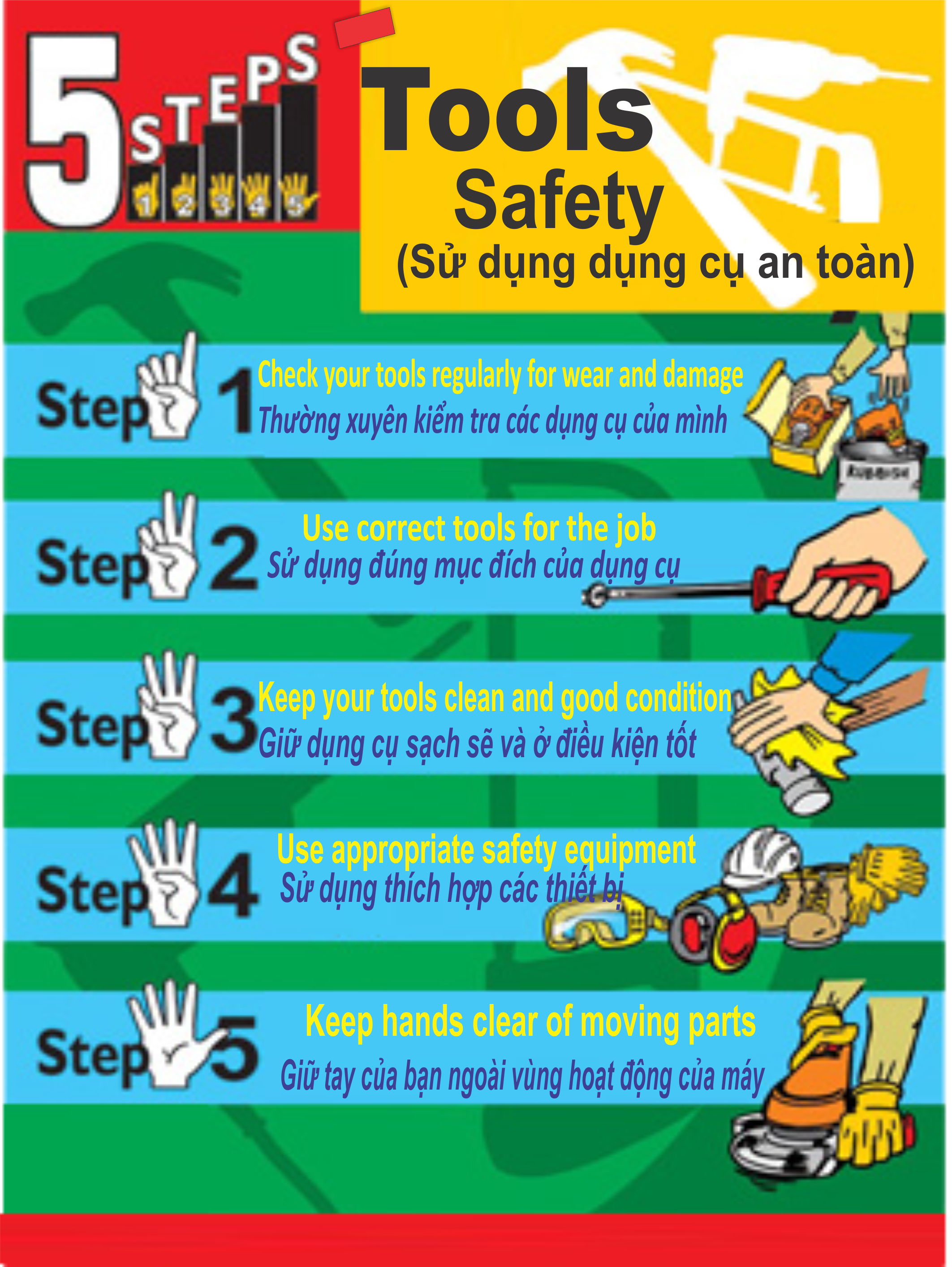






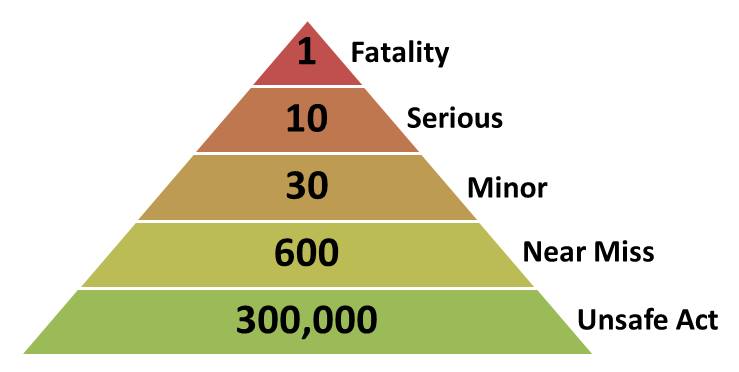
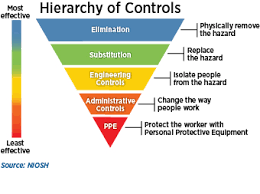





































































































.png)
